Diameter of a Hydrogen Atom in Its Ground State
Suppose an empty universe with the exception of a single hydrogen atom 1 proton 1 electron. The diameter of a hydrogen atom in its ground state is around 1 x 10-8 cm so the ratio of the diameters is about 16 x 10-5 which makes the ratio of the volumes 41 x 10-15.

Solved P9 5 In This Problem You Will Calculate The Chegg Com
The volume occupied by the electron is all but a tiny fraction of the volume of the atom while 9995 of the atoms mass is in the proton.
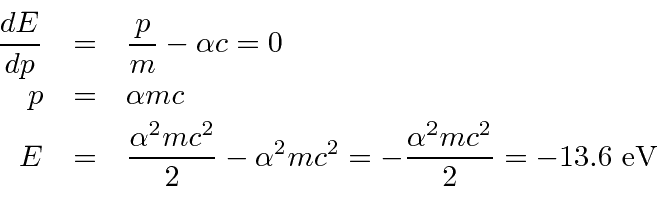
. Å is 10 times smaller than nanometer. Express the diameter of a ground-state hydrogen atom in meters using a power of 10. The lowest energy state ground state of an atom has its electrons filling orbitals beginning from lowest energy with 2 electrons per orbital.
The covalent radius is quite a lot less than the Bohr radius because hydrogen atoms in molecules tend to be. The Radius Of Hydrogen Atom In The Ground State Is The radius of hydrogen atom in the ground state is 53 x 10-11. The state of an electron in a hydrogen atom is specified by its quantum numbers n l m.
The smallest atom hydrogen has a diameter of about 1 angstrom or 01 nanometers in its ground state while the biggest atoms with around a hundred protons and an equal number of electrons are perhaps four times as big. PHYSICS An electron of kinetic energy 115 eV collides with a hydrogen atom in its ground state. Hydrogen is the simplest atoms which only contains an electron and a proton.
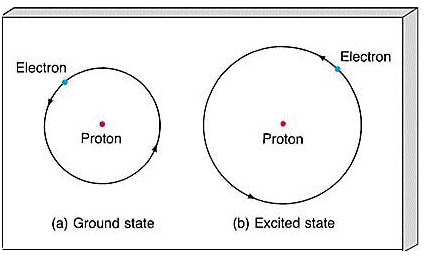
See the answer See the answer See the answer done loading. The ground state of an atomic nucleus atom or molecule is its lowest energy stateHigher energy states are described as excited states. This expression evaluates to 53 pm so from this the diameter of a hydrogen atom would be 106 pm.
Practice entering numbers that include a power of 10 by entering the diameter of a hydrogen atom in its ground state di 106 x 10 10 m into the answer box. For a hydrogen atom in its ground state use the Bohr model to compute a the orbital speed of the electron b the kinetic energy of the electron and c. The n 1 state is known as the ground state while higher n states are known as excited states.
They are provided to the right of the answer box. A simple expression for the energy of an electron in the hydrogen atom is. When hydrogen absorbs a quantity of energy exactly equal to E 1 the electron goes from the orbital in the first shell n 1 to an orbital in the second shell n 2.
1s orbital with n 1 then hydrogen atom will be in its the ground state. Express the diameter of a ground-state hydrogen atom in meters using a power of 10. E136n2 where the energy is in electron volts.
Diameter of a hydrogen atom in its ground state dh10610-11power This problem has been solved. 529 1011 m. Do not enter the units.
It also depends on the quantum state of the atom. Excited states put the electron further out and are therefore larger. 10610 10 m.
A hydrogen atom can be described in terms of its wave function probability density total energy and orbital angular momentum. The total energy of an electron in the nth orbital of a Hydrogen atom is given by E_n-dfrac136n2eV Calculation. Up to 256 cash back Get the detailed answer.
Hydrogen atom in its ground state is excited by means of a monochromatic radiation of wavelength 970. The electron may be in its ground state or it may be excited a certain number of levels. Ground state of a hydrogen atom is -136 eV.
Thus the correct option is 4. Of atoms and molecules are important as are their excited states. We define another unit of length called Angstrom Å to be 10 10 m.
Hydrogen has only one electron if it is present in the lowest energy level which is 1st energy level ie. Diameter of a hydrogen atom in its ground state dh10610-11power. Energy for n 7 state.
To convert to joules you can x this by 161019. Now the diameter will vary somewhat based on whether the atoms in its ground state. It is named after Niels.
327 1024 ℓP. The radius of hydrogen atom is ground state is 5 xx 10-11m. In contrast to the Bohr model of the atom the Schrödinger model makes predictions based on probability statements.
The ground state of hydrogen is the lowest allowed energy level and has zero angular. The diameter of the H-atom at its ground state becomes. Practice entering numbers that include a power of 10 by entering the diameter of a hydrogen atom in its ground state dH1061010m into the answer box.
The energy of n th shell is given by EdfracE_0n2 E-dfrac13649 E 028 eV. Practice entering numbers that include a power of 10 by entering the diameter of a hydrogen atom in its ground state dH1061010m d H 106 10 10 m into the answer box. Is 1s ground state.
N is the principle quantum number. The Bohr radius a0 is a physical constant approximately equal to the most probable distance between the nucleus and the electron in a hydrogen atom in its ground state. The diameter of a ground-state hydrogen atom in meters using scientific notation is 1061010.
Chemistry questions and answers. So for an electron in n1. 1 on a question.
What is the order of velocity of electron in a hydrogen atom in ground state. After collision with an electron it is found to have a radius of 212 x 10-11 m. Consider an electron in die ground state of a hydrogen atom a Calculate the expectation value of its potential energy b What is the expectation value of its kinetic energy.

Problem 10 Problem 2 24 In Textbook The Wavefunction For The Electron In A Hydrogen Atom In Its Homeworklib
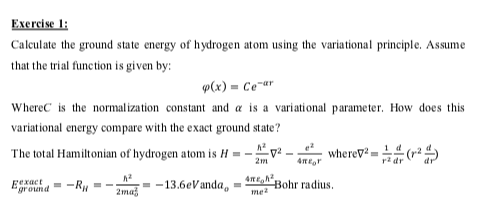
Solved Exercise 1 Calculate The Ground State Energy Of Chegg Com

Energy Of Electron In First Excited State In Hydrogen Atom Is 3 4ev Find Ke And Pe Of Electron Youtube

Solved 4 Hydrogen Atom A Consider H Atom In The Ground State The Radial Function Of A 1s Electron Is Given By 4 2 E R Ao Ris R 2 2 Calculate The Probability Of

Solved 6 For The Hydrogen Atom Z 1 In Its Ground Chegg Com
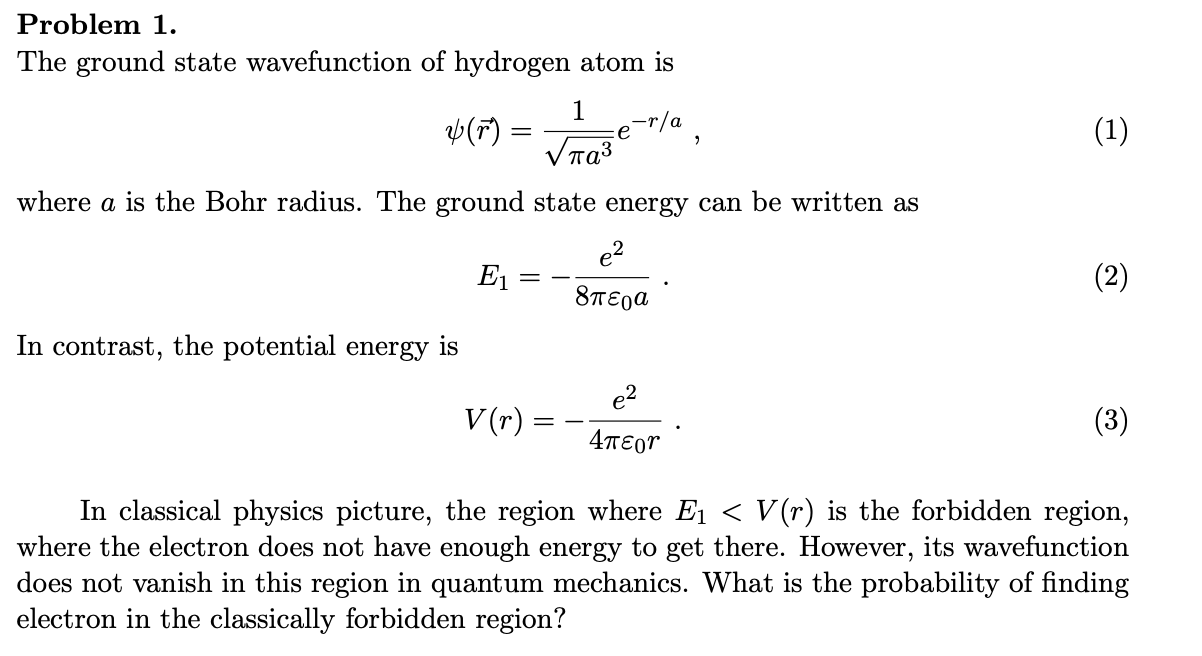
Solved Problem 1 The Ground State Wavefunction Of Hydrogen Chegg Com
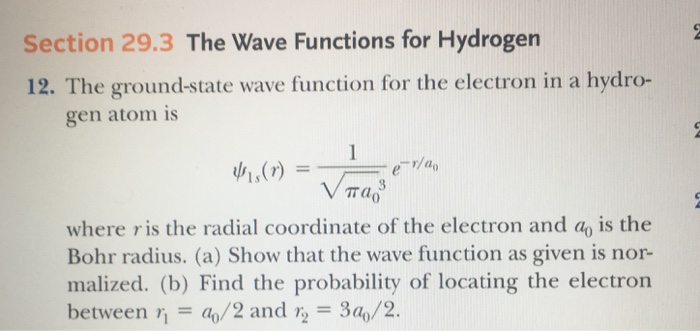
Solved The Ground State Wave Function For The Electron In A Chegg Com

The Energy Of The Electron In The Ground State Of Hydrogen Atom Is 13 6 Ev Find The Kinetic Youtube

Quantum Mechanics Ground State Of Hydrogen Atom Physics Stack Exchange
1 The First Bohr S Radius For Electron In Hydrogen Atom In The Ground State 2 The Ground Energy Level In Hydrogen Atom Ppt Video Online Download
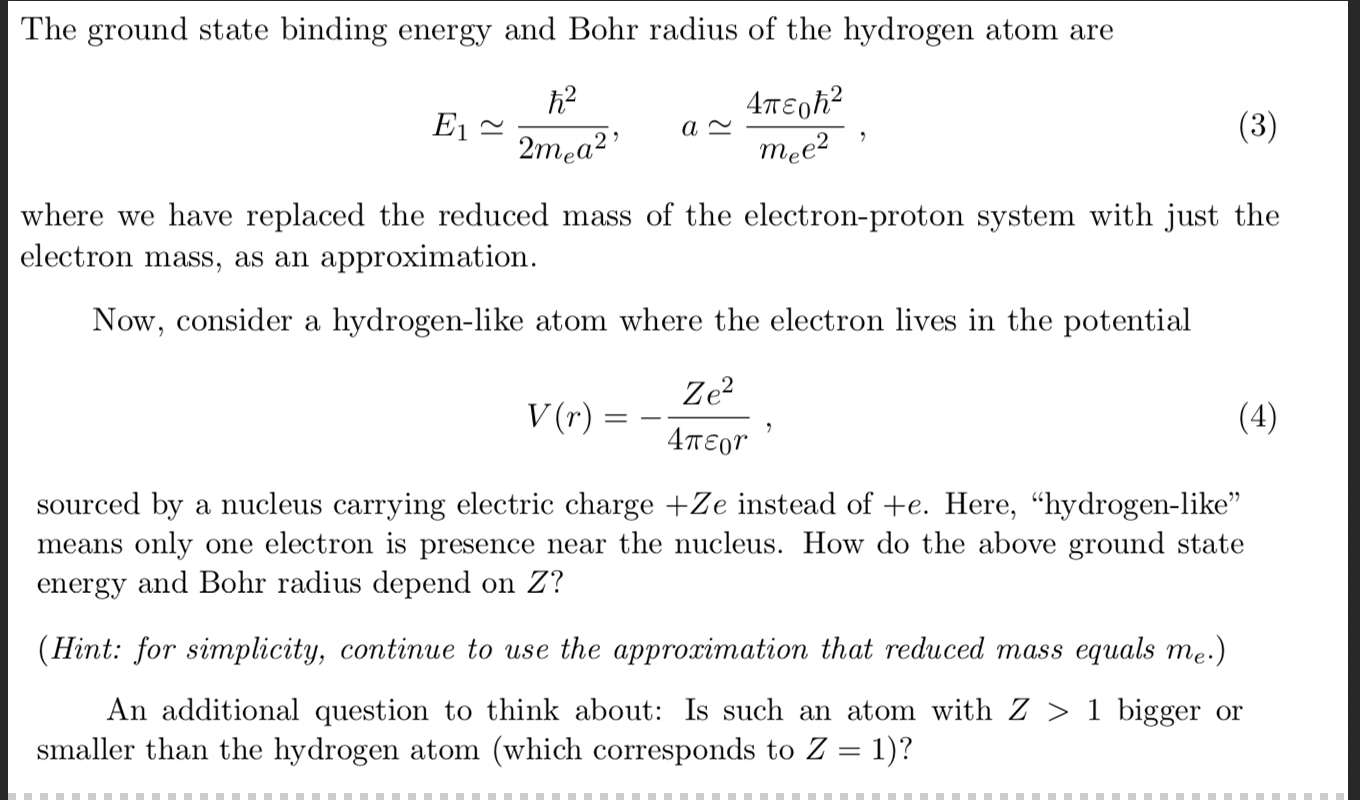
Solved The Ground State Binding Energy And Bohr Radius Of Chegg Com
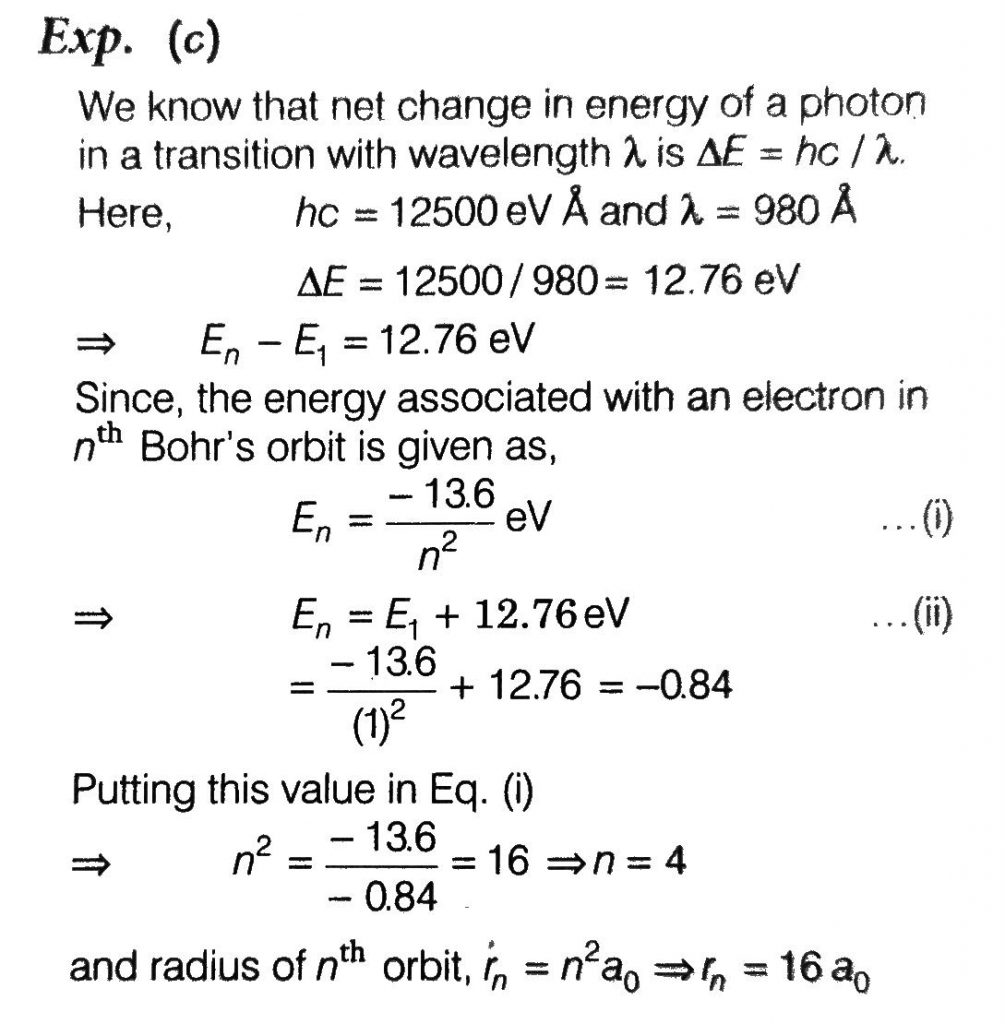
A Hydrogen Atom Initially In The Ground State Is Excited By Absorbing A Photon Of Wavelength 980a The Radius Of The Atom In The Excited State It Terms Of Bohr Radius A0

Obtain The First Bohr Radius And The Ground State Energy Of A Muonic Hydrogen Atom I E An Ato Youtube
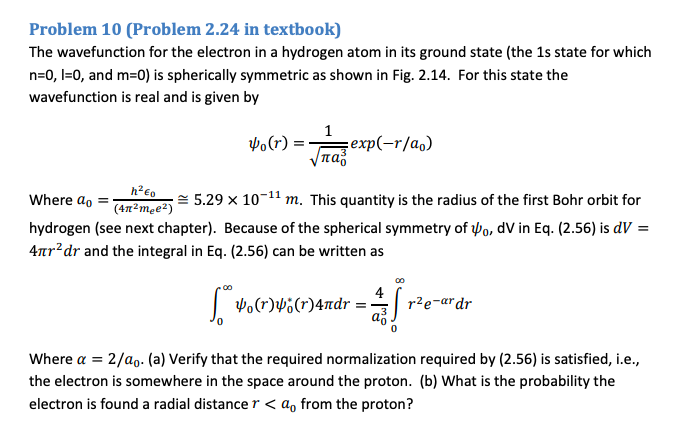
Solved Problem 10 Problem 2 24 In Textbook The Chegg Com
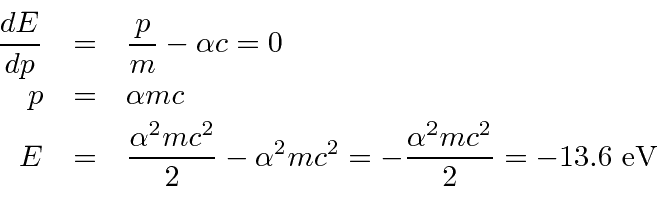
Estimate The Hydrogen Ground State Energy

The Radius Of Hydrogen Atom In Its Ground State Is 5 3 Xx 10 11 M After Collision With An Youtube

For A Hydrogen Atom In Its Ground State Use The Bohr Model To Compute A The Orbital Speed Youtube
Comments
Post a Comment